

If one neutron of Carbon 14 changes to a proton, the element becomes stable because the numbers of protons and neutrons are both seven. Carbon 14 has six protons and eight neutrons, and the state is energetically unstable because of the unbalance of both numbers. Carbon 12 is the one that most commonly exists in nature.Ĭarbon 14 is a radionuclide which exists in nature and is made through a process where a proton of Nitrogen 14 is hit and removed by a neutron created as a result of collisions of cosmic rays and the atmosphere. When calling them distinctively, they are called Carbon 11, Carbon 12, Carbon 13 and Carbon 14, adding the nuclear number (total of protons and neutrons) after the element name, which is a nominal designation that covers the same types of atoms. All of them have the same chemical properties. The nucleus is composed of protons with a positive charge and neutrons without charge, and the number of protons (atomic number) determines the chemical properties of the atom (element type).įor example, carbon has six protons, but there are also types of carbon with five, six, seven or eight neutrons. John Dalton, in 1800 proposed the first scientific.

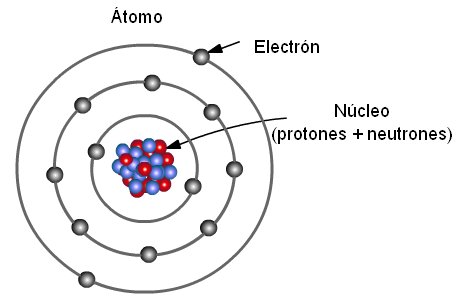
Democritus came up with the concept that matter is composed of atoms. The electrons are negatively charged particles that orbit the nucleus’s centre. An atom is composed of a nucleus and electrons that go around the former. Atomic structure is the structure of an atom that consists of a nucleus (the centre), protons (positively charged), and neutrons (neutral).


 0 kommentar(er)
0 kommentar(er)
